 |
 |
Bibliography | Home |
![]()
The excerpts below are
from Deas,
M.L. and G.T. Orlob. 1999. Klamath River Modeling Project. Project #96-HP-01.
Assessment of Alternatives for Flow and Water Quality Control in the Klamath
River below Iron Gate Dam. University of California Davis Center for Environmental
and Water Resources Engineering. Report No. 99-04. Report 236 pp. Photo is not
from Deas and Orlab (1999).
![]()
Certain elements are defined as nutrients because they are essential for life processes in aquatic organisms. Major nutrients include carbon, nitrogen, phosphorus, and silicon. Other potentially important nutrients include calcium, magnesium, sodium, potassium, and sulfur. Micronutrients, those required by plants and animals in very small quantities, might include manganese, copper, zinc, cobalt, and molybdenum (Horn and Goldman 1994). Nutrients are important in water quality for several reasons, but most often are associated with algal growth. Those critical to algal growth usually include phosphorus or nitrogen (though carbon, silicon or light limitations may play a role) (Bowie, et al 1985)
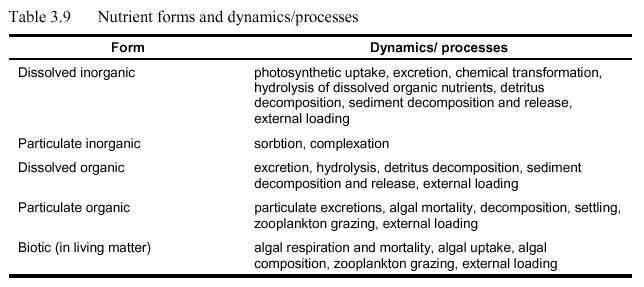
Nutrients are present in several forms in aquatic systems, including dissolved inorganic, dissolved organic, particulate organic, and biotic forms. Only dissolved forms are directly available for algal growth: for nitrogen and phosphorus these include ammonia, nitrate, nitrite, and orthophosphate (as well as dissolved CO2, and dissolved silica, etc.). Nutrient forms and selected dynamics and/or processes are included in Table 3.9. Nitrogen and phosphorus species, the nitrogen-phosphorus ratio, and impacts of nutrients on anadromous fishes are outlined below. (Please refer to original document Deas, M.L. and G.T. Orlob. 1999 for this information.)
![]()
See tables which present the recommended EPA criteria for each of the aggregate nutrient ecoregions for the following parameters: Total Phosphorus, Total Nitrogen, Chlorophyll a, and Turbidity or Secchi. Criteria are presented for both Lakes & Reservoirs and Rivers & Streams. Also, see Nitrate in Farmland Streams and Groundwater (PDF documents).
Typically, algae are autotrophic (derive cell carbon from inorganic carbon dioxide), photosynthetic (derive energy for cell synthesis from light), and contain chlorophyll. They are also chemotrophic in terms of nighttime respiration, e.g., metabolism of molecular oxygen (O2). Algae utilize photosynthesis (solar energy) to convert simple inorganic nutrients into more complex organic molecules. Photosynthetic processes results in surplus oxygen and non-equilibrium conditions by producing reduced forms of organic matter, i.e., biomass containing high-energy bonds made with hydrogen and carbon, nitrogen, sulfur, and phosphorus compounds. The organic matter produced serves as an energy source for non-photosynthetic or heterotrophic organisms (animals, including most bacteria, which subsist on organic matter). Heterotrophic organisms tend to restore equilibrium by catalytically decomposing these unstable organic products of photosynthesis, thereby obtaining a source of energy for their metabolic needs. The organisms use this energy both to synthesize new cells and to maintain old cells already formed (Stumm and Morgan, 1996). From the point of overall reactions, these heterotrophic organisms only act as reduction-oxidation catalysts - they only mediate the reaction (or more specifically the electron transfer). Oxidation may produce several intermediate reduction-oxidation states prior to reaching a fully oxidizedstate (e.g., inorganic state).
Respiration is the reverse process of growth in which protoplasm undergoes endogenous decay and/or cell lysis and oxidation. Through respiration and decomposition, organic matter is returned to the simpler (vs. complex and unstable) inorganic state. During breakdown oxygen is consumed and carbon dioxide is liberated (Chapra 1997). Although algae respire oxygen in the presence of sunlight, the amount produced via photosynthesis usually exceeds the amount used during daylight.
Light is the most limiting factor for algal growth, followed by nitrogen and phosphorus limitations. Algal productivity is often correlated to levels of nitrogen (N) and phosphorus (P) (See N:P ratio, above), but other nutrients are required including carbon, silica, and other micronutrients. Biomass is usually measured by the amount of chlorophyll a in the water column (measurement of gross level of algae) and/or as mass per area for attached species.
Chlorophyll a is a photosynthetic pigment that serves as a measurable parameter for all algae production. Quantitative biomass estimates can be made noting that on average 1.5% of algal organic matter is chlorophyll a. Qualitative assessment of primary production on water quality can be based on chlorophyll a concentrations as noted below.
| Chlr-a Concentration (g/l) | Water discoloration |
| <10 | no discoloration |
| 10-15 | some discoloration, some algal scum |
| 20-30 | deep discoloration, frequent algal scum |
| >30 | very deep discoloration, algal matting |
Though not true algae, certain strains of cyanobacteria (blue green-algae) can produce an active intracellular toxin, especially when phytoplankton are senescent (the growth phase following maturity and prior to death, characterized by accumulated metabolic products, increased respiration, and loss of dry weight) and decaying.
The intensity, duration, and quality of light influence the dominance of algal species and the structure of algal communities. Likewise, water temperature influences the metabolic and reproductive rates of algae. Although algal growth rates can be relatively lower during periods of cold water conditions, the standing crop or biomass of algal communities can be comparatively large because of the absence or inactivity of grazing organisms. Discharge and velocity conditions also affect algal communities through scouring and washout. However, modest increases in current velocity may enhance rates of algal accumulation because nutrient uptake and boundary layer diffusion increases with current velocity (Stevenson 1996). During stable hydrologic conditions, algal communities can develop in streams and rivers within several weeks of colonization and reproduction. However, such communities may vary considerably within river reaches in relation to current velocity, depth, light intensity, and water chemistry factors. Further, seasonal changes in the abundance and composition of algal communities may occur (Porter et al 1993).
Impacts on Anadromous Fish: Algal Toxicity and Algae
The role of algal toxicity to anadromous fishes is uncertain. Although algal blooms usually pose no direct health effects, certain species produce endotoxins or exotoxins that may be harmful to aquatic life. Endotoxins are of internal origin and separable from the cell body only through disintegration (e.g., death). Exotoxins are a soluble toxin produced during growth of a microorganism and released into the surrounding medium. Endotoxins can be lethal if organisms are ingested by fish, while exotoxins can cause fish kills if sufficient levels exist. Indirect algal effects, beyond toxicity, include excessive shading of the non-surface waters and subsequent reduction in photosynthetic activity, and impacts on temperature, dissolved oxygen, nutrient cycling, that may in turn affect other aspects of the ecosystem upon which salmonids may depend.
An example of indirect
impacts includes the effect of primary production on system water quality. Through
photosynthesis, algae produce oxygen in excess of respiratory requirements during
daylight hours. Conversely, during low light or nighttime periods algae respire
(consume) dissolved oxygen, sometimes depleting water column concentrations.
Thus, high algae concentrations may lead to low dissolved oxygen concentrations.
Further, during growth, algae require carbon for cell growth. Although
carbon may be present in the water column, during periods of peak growth algae
may deplete readily available forms of carbon in weakly buffered systems. When
dissolved forms are depleted carbon will enter the water column via the air-water
interface as carbon dioxide: CO2(g) ![]() .CO2(aq).
However, this process is often insufficient to keep up with algal demands. Under
such conditions certain algae species are able to utilize (remove) CO2
from bicarbonate ion: HCO3-
.CO2(aq).
However, this process is often insufficient to keep up with algal demands. Under
such conditions certain algae species are able to utilize (remove) CO2
from bicarbonate ion: HCO3-![]() CO2
+ OH -.
CO2
+ OH -.
The result is an increase in hydroxyl (OH -) concentration and an associated increase in pH. It is not uncommon to see diurnal variation in pH ranging from 0.5 to 1.5 pH units as a result of algal productivity. The increase in pH, if accompanied by elevated water temperature can cause a dramatic shift in unionized ammonia concentrations in aquatic systems. As noted previously, unionized ammonia ( NH3 vs. NH4+ ) is toxic to fish in small quantities and lethal exposure periods are on the order of hours (see Tables 3.10 through 3.12, above).
![]()
References
Bowie, G.L., W.B. Mills, D.B. Porcella, C.L. Campbell, J.R. Pagenkopf, G.L. Rupp, K.M. Johnson, P.W. Chan, and S.A. Gherini. 1985. Rates, constants and kinetics formulations in surface water quality modeling. 2Ed. EPA/600/3-85/040 U.S. Environmental Protection Agency, Environmental Research Laboratory, Athens GA.
Deas, M.L. and G.T. Orlob. 1999. Klamath River Modeling Project. Project #96-HP-01. Assessment of Alternatives for Flow and Water Quality Control in the Klamath River below Iron Gate Dam. University of California Davis Center for Environmental and Water Resources Engineering. Report No. 99-04. Report 236 pp. Appendix.
![]()
Web Sites for more information about nutrients
EPA Nutrients in Rural Waterways http://www.epa.nsw.gov.au/envirom/nutrient.htm
EPA Summary Criteria for Nutrient Enrichment http://www.epa.gov/waterscience/criteria/nutrient/ecoregions/sumtable.pdf
Nitrogen and Phosphorous http://www.heinzctr.org/ecosystems/farm_technotes/farm_nitrate_in_strms.shtml
Heinz State of the Ecosystem: http://www.heinzctr.org/ecosystems/index.htm
| www.krisweb.com |